ADC Service Workflow: From Antibody to Cytotoxic Conjugate
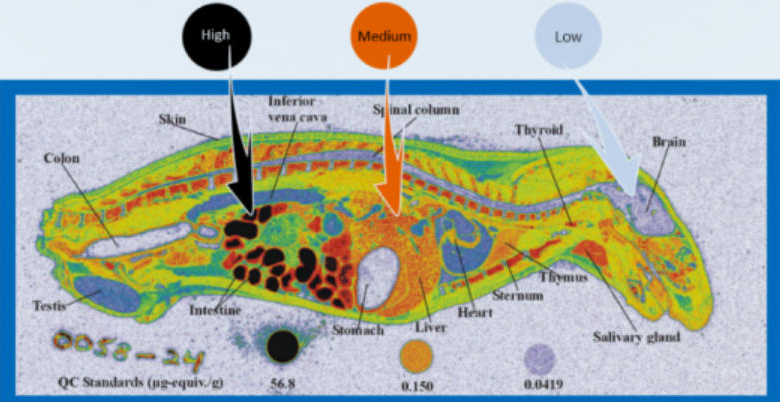
ADC service workflows describe how antibodies are transformed into targeted cytotoxic therapies through defined, connected steps. Each stage addresses specific scientific and technical risks. Understanding this workflow helps biotech teams coordinate decisions, evaluate readiness, and manage timelines. A clear view of the ADC service workflow also improves communication between discovery, development, and manufacturing partners across complex multidisciplinary programs.
Antibody Selection and Early Feasibility in ADC Services
Target Validation and Antibody Screening Strategy
ADC services begin with target validation and antibody screening. Teams confirm target expression, internalization, and disease relevance. Screening strategies compare affinity, specificity, and cross reactivity. Strong selection criteria reduce downstream failure. By identifying suitable antibodies early, adc services help developers focus resources on candidates with a higher likelihood of effective payload delivery and acceptable safety margins during later development stages.
Antibody Engineering for Conjugation Readiness
Antibody engineering prepares molecules for efficient conjugation. ADC services modify sequences to improve stability, reduce aggregation, or introduce conjugation sites. These adjustments maintain binding while supporting controlled drug attachment. Engineering for conjugation readiness prevents later rework and supports consistent manufacturing. Early optimization ensures antibodies can tolerate chemical modification without losing functional or developability properties critical for downstream success.
Early Developability and Feasibility Assessment
Early developability assessments evaluate whether antibody designs can progress smoothly. ADC services examine expression yield, purity, stability, and compatibility with payload chemistry. Feasibility studies identify risks before heavy investment. This step protects timelines by eliminating weak candidates early and ensuring remaining programs move forward with realistic expectations regarding scalability, quality, and integration into full ADC development workflows.
Payload and Linker Design Within ADC Service Workflows
Cytotoxic Payload Selection Criteria
Payload selection defines therapeutic impact and safety profile. ADC services evaluate cytotoxic mechanisms, potency ranges, and resistance risks. Selection criteria align payload strength with target biology and dosing strategy. Choosing the right payload avoids excessive toxicity while maintaining efficacy. Structured evaluation helps teams avoid overpowered designs and supports balanced candidates suitable for controlled delivery through antibody targeting systems.
Linker Chemistry and Release Mechanisms
Linker chemistry controls when and where payloads are released. ADC services assess cleavable and non cleavable linkers, considering stability in circulation and release inside target cells. Release mechanisms must match disease context. Well chosen linkers prevent premature payload loss and improve therapeutic precision. This step directly influences safety margins and consistency of in vivo performance.
Balancing Potency, Stability, and Safety
ADC service workflows aim to balance potency, stability, and safety. High potency alone does not ensure success. Services integrate antibody behavior, linker design, and payload strength to manage risk. Balanced designs reduce off target effects and improve tolerability. This holistic evaluation supports candidates that perform reliably across preclinical models and align with long term clinical development goals.
Conjugation and Process Development Services
Conjugation Methods and Drug-to-Antibody Ratio Control
Conjugation methods determine ADC uniformity and performance. ADC services select chemical or site specific approaches to control to drug-to-antibody ratio. Tight DAR control improves consistency and predictability. Experienced teams optimize reaction conditions to minimize variability. Reliable conjugation supports accurate data interpretation and establishes a solid foundation for scale up and regulatory confidence.
Process Optimization for Consistency and Yield
Process optimization focuses on achieving consistent output and acceptable yield. ADC services refine parameters such as reaction time, temperature, and purification steps. Optimized processes reduce batch variability and material loss. Consistency improves comparability across studies. Early optimization prevents delays and supports smoother transitions into larger scale production environments required for expanded preclinical programs.
Scale-Up Considerations for Preclinical Supply
Scaling up for preclinical supply introduces new challenges. ADC services adapt small scale processes to larger batches while maintaining quality. Teams assess equipment compatibility, raw material availability, and reproducibility. Addressing scale up early avoids supply gaps. Reliable preclinical supply supports continuous in vivo studies and keeps development timelines aligned with internal and external milestones.
Analytical and Preclinical Validation in ADC Services
Structural Characterization and Quality Assessment
Structural characterization confirms ADC identity and quality. ADC services apply analytical tools to assess purity, aggregation, DAR distribution, and integrity. Quality assessment ensures material consistency across studies. Clear analytical data support confident interpretation of biological results. This step links chemistry and biology, ensuring observed effects reflect molecular design rather than uncontrolled quality variation.
In Vitro and In Vivo Preclinical Evaluation
Preclinical evaluation tests whether ADCs perform as intended. ADC services coordinate in vitro assays and in vivo models to assess potency, exposure, and safety. Aligned study design improves data relevance. Integrated evaluation helps teams confirm mechanisms, understand dose response, and identify limitations before committing to more extensive regulatory enabling activities.
Data Integration for Candidate Advancement Decisions
Data integration connects results from multiple workflow stages. ADC services compile analytical, biological, and process data into coherent summaries. Integrated views highlight strengths and risks clearly. This clarity supports advancement decisions based on evidence rather than assumptions. Well organized data packages also facilitate internal reviews, partner discussions, and preparation for subsequent development phases.
Conclusion
ADC service workflows provide a structured path from antibody selection to cytotoxic conjugate validation. Each step addresses specific technical risks and informs better decisions. Understanding this workflow helps biotech teams plan resources, manage timelines, and improve outcomes. Clear, connected ADC service workflows strengthen candidate quality and support efficient progression toward preclinical and clinical development goals.





